Difference between revisions of "Choline Bitartarate"
From DolceraWiki
(→Action of Choline bitartrate containing Compositions) |
(→Dashboard) |
||
| (24 intermediate revisions by 3 users not shown) | |||
| Line 7: | Line 7: | ||
* As B vitamins are water soluble, they are excreted in the urine and can be quickly depleted from the body. Only a small amount is stored in the body. Because of this, it is important that we take supplements to replenish these important vitamins in our body. [http://www.add-adhd-help-center.com/Ingredients/choline_bitartrate.htm Source] | * As B vitamins are water soluble, they are excreted in the urine and can be quickly depleted from the body. Only a small amount is stored in the body. Because of this, it is important that we take supplements to replenish these important vitamins in our body. [http://www.add-adhd-help-center.com/Ingredients/choline_bitartrate.htm Source] | ||
| − | * Choline bitartrate is an essential nutrient needed by the nervous system to produce acetylcholine. Acetylcholine is a neurotransmitter that facilitates the transmission of impulses between neurons. | + | * Choline bitartrate is an essential nutrient needed by the nervous system to produce acetylcholine. Acetylcholine is a neurotransmitter that facilitates the transmission of impulses between neurons. [http://www.ncbi.nlm.nih.gov/pubmed/14972364 Source] |
'''Natural Source of Choline bitartrate''': Egg yolk, peanuts, wheat germ, organ meats and legumes. | '''Natural Source of Choline bitartrate''': Egg yolk, peanuts, wheat germ, organ meats and legumes. | ||
| Line 52: | Line 52: | ||
|Combine | |Combine | ||
|align = "center"|'''1AND 2''' | |align = "center"|'''1AND 2''' | ||
| − | |align = "center"|''' 881 hits (Unique Records | + | |align = "center"|''' 881 hits (Unique Records 444)''' |
|- | |- | ||
|} | |} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ===='''STN Search Strategy'''==== | ||
| + | =<nowiki>></nowiki> FILE REGISTRY | ||
| + | |||
| + | =<nowiki>></nowiki> UPLOAD STRUCTURE | ||
| + | |||
| + | '''L1''' | ||
| + | |||
| + | [[Image:Cholinebitartrate.jpg|thumb|center|750px|'''Cholinebitartrate''']] | ||
| + | |||
| + | =<nowiki>></nowiki> D L1 | ||
| + | |||
| + | =<nowiki>></nowiki> S L1 EXACT SAM | ||
| + | |||
| + | '''L2''' | ||
| + | |||
| + | =<nowiki>></nowiki> D SCAN | ||
| + | |||
| + | =<nowiki>></nowiki> S L1 EXACT FULL | ||
| + | |||
| + | '''L3''' | ||
| + | |||
| + | SET PLURAL ON PERM | ||
| + | |||
| + | SET ABBREVIATION ON PERM | ||
| + | |||
| + | SET SPELLINGS ON PERM | ||
| + | |||
| + | =<nowiki>></nowiki> S L3 AND PATENT/DT | ||
| + | |||
| + | '''L4''' | ||
| + | |||
| + | =<nowiki>></nowiki> S L3 NOT PATENT/DT | ||
| + | |||
| + | '''L5''' | ||
| + | |||
| + | =<nowiki>></nowiki> D L4 IALL 1- | ||
| + | |||
| + | =<nowiki>></nowiki> D L5 IALL 1- | ||
| + | |||
| + | =<nowiki>></nowiki> '''LOGOFF Y''' | ||
| + | |||
| + | ---- | ||
===Analysis Taxonomy=== | ===Analysis Taxonomy=== | ||
| Line 67: | Line 113: | ||
====Top Assignees==== | ====Top Assignees==== | ||
| − | *Council of Scientific & Industrial Research found to be the Top | + | *Council of Scientific & Industrial Research found to be the Top assignees with 3 patents. |
| − | * For 2 patent / publications | + | * For 2 patent / publications Assignee name is not available. |
[[Image:CBT TOP assignee.jpg|center|600 px|thumb|Graph showing Top assignee names]] | [[Image:CBT TOP assignee.jpg|center|600 px|thumb|Graph showing Top assignee names]] | ||
| Line 81: | Line 127: | ||
====Action of Choline bitartrate containing Compositions==== | ====Action of Choline bitartrate containing Compositions==== | ||
| − | <mm>[[ | + | <mm>[[Choline_bitartrate.mm]]</mm> |
====Choline Bitartarate Patents categorised as per formulation==== | ====Choline Bitartarate Patents categorised as per formulation==== | ||
| Line 186: | Line 232: | ||
====Choline Bitartarate containing compositions==== | ====Choline Bitartarate containing compositions==== | ||
| − | * | + | *Composition components matrix showing all the ingredients used along with Choline Bitartarate. |
| − | *'''[[Media:cbt | + | {|border="2" cellspacing="0" cellpadding="4" width="100%" |
| + | |align = "center" bgcolor = "#FFCC99" colspan = "13"|<font size = "1">'''Beverage / liquid / Drink'''</font> | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Patent/Publication No.'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Amino acids'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Antioxidant'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Carbohydrates / Sugars'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Energy Source / Enhancer'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Fatty Acids / A lipid Source'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Herbal Extracts'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Minerals'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Proteins'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Stimulant'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Vitamins'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Vegetable Sources'''</font> | ||
| + | |align = "center" bgcolor = "#CCFFCC"|<font size = "1">'''Others'''</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220040005368%22.PGNR.&OS=DN/20040005368&RS=DN/20040005368 US20040005368A1]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1">Caffeine, Green Tea</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Oxygen uptake enhancing substance.</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220040191294%22.PGNR.&OS=DN/20040191294&RS=DN/20040191294 US20040191294A1]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Milk or milk-based products</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220060121158%22.PGNR.&OS=DN/20060121158&RS=DN/20060121158 US20060121158A1]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Flavanol</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=5626849.PN.&OS=PN/5626849&RS=PN/5626849 US5626849A]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">gamma-linolenic acid</font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">L-carnitine</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=5904948.PN.&OS=PN/5904948&RS=PN/5904948 US5904948A]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Maltodextrin</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1">Whey , milk or vegetable</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://v3.espacenet.com/textdoc?DB=EPODOC&IDX=WO2006052231&F=0 WO2006052231A1]</u></font> | ||
| + | |<font size = "1">leucine, isoleucine, valine and arginine.</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220050171034%22.PGNR.&OS=DN/20050171034&RS=DN/20050171034 US20050171034A1]</u></font> | ||
| + | |<font size = "1">N-acetyl-cysteine</font> | ||
| + | |<font size = "1">Alpha-lipoic acid</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Zn</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">A, B, C, E</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Betaine</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220060241077%22.PGNR.&OS=DN/20060241077&RS=DN/20060241077 US20060241077A1]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Uridine or its derivative</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220070065456%22.PGNR.&OS=DN/20070065456&RS=DN/20070065456 US20070065456A1]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Green tea extract</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Taurine</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Wasabia japonica, Silybum marianum and Cynara scolymus.</font> | ||
| + | |<font size = "1">Mn, Se Zn</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220070248696%22.PGNR.&OS=DN/20070248696&RS=DN/20070248696 US20070248696A1]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">B1,B5, B6, B12,folic acid</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Dimethylaminoethanol, cytidine 5<nowiki>’</nowiki>-diphosphocholine, turmeric extract, Green tea extract</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220080317868%22.PGNR.&OS=DN/20080317868&RS=DN/20080317868 US20080317868A1]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Nucleotide fraction</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=4497800.PN.&OS=PN/4497800&RS=PN/4497800 US4497800A]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=4499076.PN.&OS=PN/4499076&RS=PN/4499076 US4499076A]</u></font> | ||
| + | |<font size = "1">Essential AA<nowiki>’</nowiki>S</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1"> </font> | ||
| + | |<font size = "1">Calcium pantothenate, nicotinic acid amide, biotin, folic acid</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=4837219.PN.&OS=PN/4837219&RS=PN/4837219 US4837219A]</u></font> | ||
| + | |<font size = "1">L-Tyrosine, L-Phenylalanime, L-Leucine</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Zn and Cu</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=5437880.PN.&OS=PN/5437880&RS=PN/5437880 US5437880A]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1">Digestible saccharide</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">A B, C, D, E and K .</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Carotenoids</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://v3.espacenet.com/textdoc?DB=EPODOC&IDX=WO9415488&F=0 WO1994015488A2]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |align = "center"|<font size = "1">x</font> | ||
| + | |<font size = "1">choline Bitartrate</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Neuromuscular function enhancer</font> | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#FFCC99" colspan = "13"|<font size = "1">'''Foodstuff'''</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PG01&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.html&r=1&f=G&l=50&s1=%2220050208191%22.PGNR.&OS=DN/20050208191&RS=DN/20050208191 US20050208191A1]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">x</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">Fe, Zn, Cu, I ,</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">x</font> | ||
| + | |<font size = "1">Sesame seed paste Sesame seed</font> | ||
| + | |<font size = "1">Whole wheat, Roasted (defatted) soy Peanut paste, Wheat germ (roasted), Non fat dry milk</font> | ||
| + | |- | ||
| + | |<font color="#0000FF"><u>[http://v3.espacenet.com/textdoc?DB=EPODOC&IDX=WO2005087018&F=0 WO2005087018A1]</u></font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">x</font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1"> </font> | ||
| + | |<font size = "1">x</font> | ||
| + | |<font size = "1">Wheat flour, roasted peanut paste, sesame seed, soybean flour</font> | ||
| + | |<font size = "1">x</font> | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | |||
| + | *'''[[Media:cbt composition matrix.xls|Click here]]''' for Composition matrix showing all the ingredients used along with Choline Bitartarate. | ||
===Patent Analysis Sheets=== | ===Patent Analysis Sheets=== | ||
| − | *'''[[Media:cbt Patent Analysis sheet.xls|Click here for the complete Analysis spread sheet | + | *'''[[Media:All Patent Analysis sheet.xls|Click here for all Patents spread sheet - Total count: 444]]''' |
| + | |||
| + | *'''[[Media:cbt Patent Analysis sheet.xls|Click here for the complete Analysis spread sheet ON Target count: 22]]''' | ||
* '''[[Media:Other than cbt ingredients.xls|Click here]]''' for list of ingredients that are in combination with Choline Bitartarate, obtained from Patents. | * '''[[Media:Other than cbt ingredients.xls|Click here]]''' for list of ingredients that are in combination with Choline Bitartarate, obtained from Patents. | ||
| Line 216: | Line 538: | ||
==Dashboard== | ==Dashboard== | ||
| − | * Click at this link for '''[ | + | * Click at this link for '''[https://www.dolcera.com/auth/dashboard/dashboard.php?workfile_id=554 Dashboard]''' |
Latest revision as of 03:48, 27 July 2015
Contents
Overview
- Choline is an organic compound, usually grouped within the Vitamin B complex. There are eight B vitamins in the Vitamin B complex family. Although each performs a different function in the body, they all work together to maintain good health.
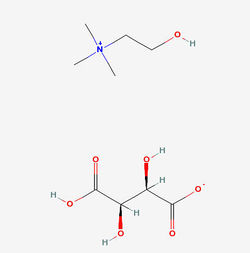
Structure of Choline bitartrate Source
- Choline is available in various forms namely, choline bitartrate, choline citrate,choline chloride and others.
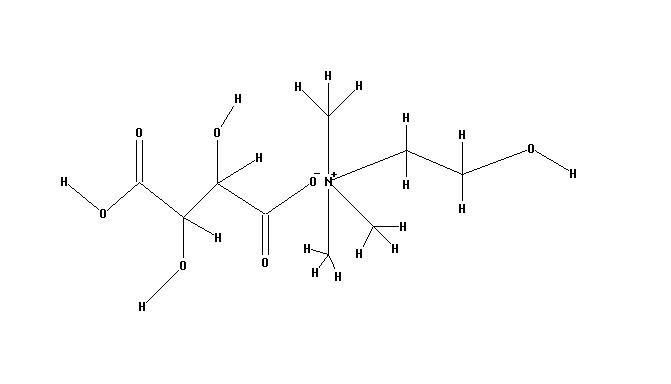
- Choline Bitartrate (L(+) choline bitartrate) is a colorless or white crystal powder, which is a water-soluble part of the B Vitamin family. Its IUPAC Name: 2-hydroxyethyl(trimethyl)azanium; (2R,3R)-2,3,4-trihydroxy-4-oxobutanoate and CAS Registry Number is : 87-67-2. Source
- The body needs B vitamins to manufacture neurotransmitters, chemicals that control alertness and mood by speeding nerve signals through the brain.
- As B vitamins are water soluble, they are excreted in the urine and can be quickly depleted from the body. Only a small amount is stored in the body. Because of this, it is important that we take supplements to replenish these important vitamins in our body. Source
- Choline bitartrate is an essential nutrient needed by the nervous system to produce acetylcholine. Acetylcholine is a neurotransmitter that facilitates the transmission of impulses between neurons. Source
Natural Source of Choline bitartrate: Egg yolk, peanuts, wheat germ, organ meats and legumes.
Health Benefits of Choline Bitartrate:
- Being a product of vitamin, it can be applied to medicine, healthcare product and food.
- It is a nutritious additive and fat remover, enhance fatty metabolism and eliminate the accumulation of fat in liver.
- Influence muscle contractions, movement, coordination and enhance memory.
- Involved in higher level brain functions like memory, thought and intellect.
- Is vital to the structural integrity of cell walls, the production of amino acids and proteins and the metabolism of fats.
- Aid in the treatment of Alzheimer’s Disease, manic depression and improve the symptoms of Parkinson’s Disease.
Deficiencies in B vitamins causes:
- Depression or signs of decreased mental functioning.
- Decrease cognitive function.
Intellectual Property
Search Strategy
- Databases: USG USA EPA EPB WO JP DEG DEA DET DEU GBA FRA
- Years: From 1836 - To April 18, 2009.
| S.No | Concept | Scope | Search Query | Hits |
| 1 | Choline bitartarate | Full Spec. | ((choline ADJ1 bitartarate*) OR (choline ADJ1 bitartrate*) OR (choline ADJ1 bi ADJ1 tartrate*) OR (2-Hydroxyethyl ADJ1 trimethyl ADJ1 ammonium ADJ1 bitartrate) OR ((2-Hydroxyethyl) ADJ1 trimethyl ADJ1 ammonium ADJ1 bitartrate) OR ((2-Hydroxyethyl)trimethylammonium ADJ1 bitartrate)) | 1016 hits |
| 2 | Food & beverage | Claims, Title or Abstract | (beverage* OR drink* OR juice* OR potion OR tonic OR spirit* OR (liquid ADJ1 refreshment) OR Tea OR milk OR coffee OR cocoa OR (liquid ADJ (formulation*1 OR preparation*1)) OR capsule* OR caplet* OR tablet* OR powder*2 OR (Nutri* ADJ supplement*) OR Food* OR Meal* OR composition OR (Nutrition* NEAR3 adjuvant* ) OR (food NEAR3 supplement*) OR formula OR formulation*) | 3677912 hits |
| 3 | Combine | 1AND 2 | 881 hits (Unique Records 444) |
STN Search Strategy
=> FILE REGISTRY
=> UPLOAD STRUCTURE
L1
=> D L1
=> S L1 EXACT SAM
L2
=> D SCAN
=> S L1 EXACT FULL
L3
SET PLURAL ON PERM
SET ABBREVIATION ON PERM
SET SPELLINGS ON PERM
=> S L3 AND PATENT/DT
L4
=> S L3 NOT PATENT/DT
L5
=> D L4 IALL 1-
=> D L5 IALL 1-
=> LOGOFF Y
Analysis Taxonomy
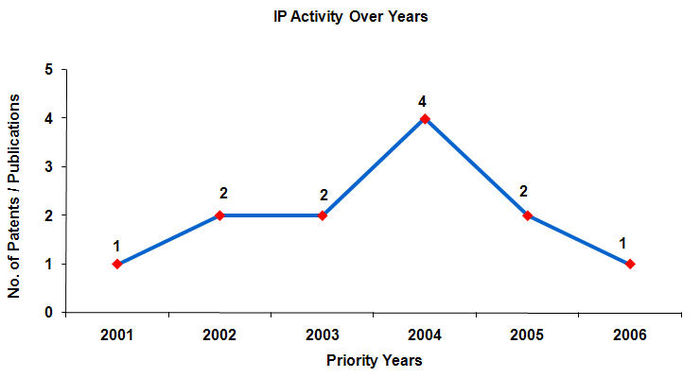
IP activity over the years
- Maximum number of patents have been filed in the year 2004.
- Patents are published 18 months after application.
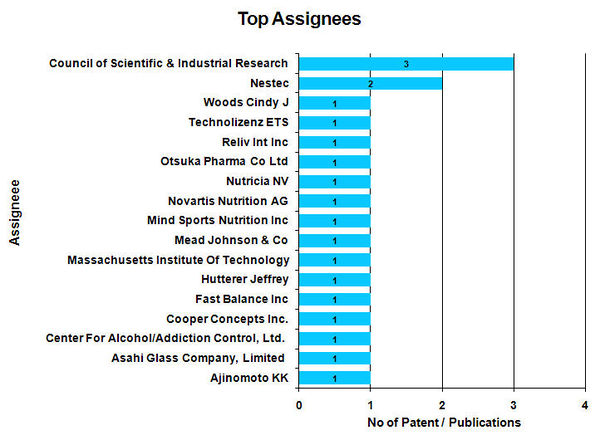
Top Assignees
- Council of Scientific & Industrial Research found to be the Top assignees with 3 patents.
- For 2 patent / publications Assignee name is not available.
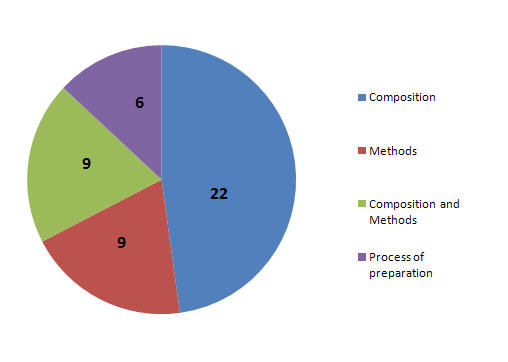
Patent Focus
- Patents focusing on Composition.
- Patents focusing on Methods.
- Patents focusing on Composition and Methods.
- Patents focusing on process of prepration.
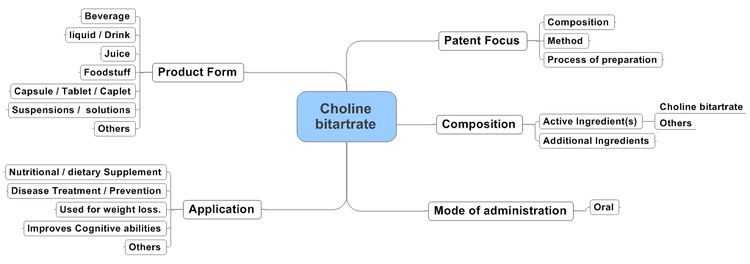
Action of Choline bitartrate containing Compositions
Choline Bitartarate Patents categorised as per formulation
| Product form | Patent/publication | Patent focus | Ingredients other than Choline Bitartarate | Application |
| Beverage / liquid / Drink | US20040005368A1 | Composition and Method | 1. At least one substance that enhance oxygen uptake such as Gingko A, Caffeine, Green Tea, L-pyroglutamate, Xanthinol nicotinate. 2. A protein supplement. |
Used for weight loss. |
| US20040191294A1 | Composition, Method, Process of prepration | 1. one or more omega 3 fatty acids. 2. milk or milk-based products . |
lowers levels of cholesterol and triglycerides. | |
| US20060121158A1 | Composition | 1. A first ingredient comprising at least one of a polyol, a fiber or a combination thereof. 2. Second ingredient comprising calcium. 3. Third ingredient comprising at least one of flavanol, stimulant and antioxidant. |
Used for weight management. | |
| US5626849A | Composition and Method | An essentially dry mixture of chromium, L-carnitine, gamma-linolenic acid, (-) hydroxycitric acid, inositol, antioxidants and herbs. | A dietary supplement to help facilitate weight loss | |
| US5904948A | Composition and Process of prepration | An protein component and a carbohydrate component. | Nutritional Supplement | |
| WO2006052231A1 | Composition and Process of prepration | Sugar including the carbohydrates, fructose and maltodextrin, and electrolytes chromium, copper, potassium, magnesium, sodium, and citric acid. | Restoring human growth hormone. | |
| US20050171034A1 | Composition and Method | Betaine, pyridoxal-5-phosphate, and N-acetyl-cysteine. | Promotes decrease of homocysteine levels in humans. | |
| US20060241077A1 | Composition and Method | A uridine, an acyl derivative thereof, a uridine phosphate, uracil, or a salt thereof; | Ameliorating or inhibiting decline in memory or intelligence. | |
| US20070065456A1 | Composition | Wasabia japonica, Silybum marianum, Cynara scolymus. | Nutritional supplement for a human diet. | |
| US20070248696A1 | Composition and Method | Dimethylaminoethanol, cytidine 5’-diphosphocholine, turmeric extract, decaffeinated green tea extract, vitamin B1,B5, B6, B12,folic acid, Dimethylglycine, huperzine A, Griffonia simplicifolia extract and 1-phenylalanine. | Improves neuromuscular facilitation and enhances cognitive functions, such as memory and mental focus. | |
| US20080317868A1 | Composition | An energy content, a protein fraction, a lipids fraction, a nucleotide fraction and a mineral fraction. | An anti-allergic infant formulation. | |
| US4497800A | Composition | A protein equivalent, a suitable lipid, a carbohydrate component , essential vitamins , minerals and an emulsion stabilizer. | Providing nourishment for a human patient in need of such nourishment . | |
| US4499076A | Composition | Essential amino acids, carbohydrates, fats, vitamins namely vitamin A, B1, B6, B12, C, D2, E, K1, calcium pantothenate, nicotinic acid amide, biotin, folic acid or choline bitartrate and minerals. | New elemental diets for liver diseases. | |
| US4837219A | Composition, Method, Process of prepration | L-Tyrosine, L-Phenylalanime, L-Leucine, Zinc (from Zinc Gluconate) and Copper (from Copper Gluconate). | A medication for Alzheimer’s disease. | |
| US5437880A | Composition | Digestible saccharide and a carotenoid. | A health drink | |
| WO1994015488A2 | Composition | Carbohydrate, electrolyte, ammonia neutralizer, energy enhancer namely choline Bitartrate and others , antioxidant, neuromuscular function enhancer. | ||
| Foodstuff | US20050208191A1 | Composition and Process of prepration | Whole wheat, Roasted (defatted) soy Peanut paste, Sesame seed paste Sesame seed , Wheat germ (roasted), Non fat dry milk, Sugar Powder, Liquid, Lecithin, Sodium Chloride, mmonium bicarbonate, vitamins and others. | Nutritious baked snack food with high protein content |
| WO2005087018A1 | Composition and Process of prepration | vegetable sources as wheat flout, roasted peanut paste, sesame seed, soybean flour and well balanced mixture of vitamins, minerals and others. | Food stuff for children and adult supplementing their nutritional requirement. |
Choline Bitartarate containing compositions
- Composition components matrix showing all the ingredients used along with Choline Bitartarate.
| Beverage / liquid / Drink | ||||||||||||
| Patent/Publication No. | Amino acids | Antioxidant | Carbohydrates / Sugars | Energy Source / Enhancer | Fatty Acids / A lipid Source | Herbal Extracts | Minerals | Proteins | Stimulant | Vitamins | Vegetable Sources | Others |
| US20040005368A1 | x | Caffeine, Green Tea | Oxygen uptake enhancing substance. | |||||||||
| US20040191294A1 | x | Milk or milk-based products | ||||||||||
| US20060121158A1 | x | x | x | x | x | Flavanol | ||||||
| US5626849A | x | gamma-linolenic acid | x | L-carnitine | ||||||||
| US5904948A | Maltodextrin | x | Whey , milk or vegetable | x | ||||||||
| WO2006052231A1 | leucine, isoleucine, valine and arginine. | x | x | |||||||||
| US20050171034A1 | N-acetyl-cysteine | Alpha-lipoic acid | Zn | A, B, C, E | Betaine | |||||||
| US20060241077A1 | Uridine or its derivative | |||||||||||
| US20070065456A1 | Green tea extract | Taurine | Wasabia japonica, Silybum marianum and Cynara scolymus. | Mn, Se Zn | x | x | ||||||
| US20070248696A1 | B1,B5, B6, B12,folic acid | Dimethylaminoethanol, cytidine 5’-diphosphocholine, turmeric extract, Green tea extract | ||||||||||
| US20080317868A1 | x | x | x | x | Nucleotide fraction | |||||||
| US4497800A | x | x | x | x | x | |||||||
| US4499076A | Essential AA’S | x | x | x | Calcium pantothenate, nicotinic acid amide, biotin, folic acid | x | ||||||
| US4837219A | L-Tyrosine, L-Phenylalanime, L-Leucine | Zn and Cu | x | |||||||||
| US5437880A | x | Digestible saccharide | A B, C, D, E and K . | Carotenoids | ||||||||
| WO1994015488A2 | x | x | choline Bitartrate | Neuromuscular function enhancer | ||||||||
| Foodstuff | ||||||||||||
| US20050208191A1 | x | Fe, Zn, Cu, I , | x | Sesame seed paste Sesame seed | Whole wheat, Roasted (defatted) soy Peanut paste, Wheat germ (roasted), Non fat dry milk | |||||||
| WO2005087018A1 | x | x | Wheat flour, roasted peanut paste, sesame seed, soybean flour | x | ||||||||
- Click here for Composition matrix showing all the ingredients used along with Choline Bitartarate.
Patent Analysis Sheets
- Click here for list of ingredients that are in combination with Choline Bitartarate, obtained from Patents.
Scientific Literature Analysis
| S.No. | Title | Citations | Publication Date | Results / Statastical data | Dolcera Summary |
| 1 | Effect of choline supplementation on fatigue in trained cyclists. | Med Sci Sports Exerc. 1995 May;27(5):668-73. | 1995-05-01 | 1. Twenty male cyclists (ages 23-29) with maximal aerobic power (VO2max) between 58 and 81 ml.min-1.kg-1 were randomly divided into BRIEF (N = 10) and PROLONGED (N = 10) groups. 2. One hour after drinking a beverage with or without choline bitartrate (2.43 g), cyclists began riding at a power output equivalent to approximately 150% (BRIEF) and 70% (PROLONGED) of VO2max at a cadence of 80-90 rpm. 3.Time to exhaustion, indirect calorimetry and serum choline, lactate, and glucose were measured. |
Trained cyclists do not deplete choline during supramaximal brief or prolonged submaximal exercise, nor do they benefit from choline supplementation to delay fatigue under given conditions. |
Dashboard
- Click at this link for Dashboard