Difference between revisions of "Olympus Corporation- Company Profile"
(→Revenue) |
(→Organisation Structure) |
||
| (18 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
OLYMPUS CORPORATION, a Japan-based manufacturer, mainly manufactures and sells medical products, life and industrial products, imaging products, information communication products and other products. The Company operates in five business segments. | OLYMPUS CORPORATION, a Japan-based manufacturer, mainly manufactures and sells medical products, life and industrial products, imaging products, information communication products and other products. The Company operates in five business segments. | ||
| − | * | + | * '''Medical Systems:''' Manufacture and sale of medical endoscopes, surgical endoscopes, endoscope disposal equipment and ultrasonic endoscopes |
| − | * | + | * '''Life and Industrial:''' Provides biological microscopes, industrial microscopes, endoscopes for industrial use and non-destructive testing equipment |
| − | * | + | * '''Imaging:''' Manufacture and sale of digital cameras and voice recorders |
| − | * | + | * '''Information Communication:''' Sale of mobile terminals, such as cellular phones |
| − | * | + | * '''Others:''' Development of biomaterials and systems, industrial endoscopes, nondestructive inspection equipment, printers, mobile solutions and mobile contents services, the development and sale of package software, the sale of semiconductor-related equipment and electronic machines, as well as the development of systems |
<br> | <br> | ||
| Line 14: | Line 14: | ||
===Key Facts=== | ===Key Facts=== | ||
{|border="2" cellspacing="0" cellpadding="4" width="54%" align="center" | {|border="2" cellspacing="0" cellpadding="4" width="54%" align="center" | ||
| − | |bgcolor = "# | + | |bgcolor = "#95B3D7"|'''Established''' |
|1919 | |1919 | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#95B3D7"|'''Headquarters''' |
|Tokyo, Japan | |Tokyo, Japan | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#95B3D7"|'''Type''' |
|Public Company | |Public Company | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#95B3D7"|'''Website''' |
|[http://www.olympus-global.com/en/ www.olympus-global.com] | |[http://www.olympus-global.com/en/ www.olympus-global.com] | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#95B3D7"|'''Regional involvement''' |
|Worldwide | |Worldwide | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#95B3D7"|'''Business lines''' |
|Manufacture and Sales of precision machinery and instruments | |Manufacture and Sales of precision machinery and instruments | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#95B3D7"|'''Employees''' |
|34,391<br> Medical System Business: 15,000 Approx. | |34,391<br> Medical System Business: 15,000 Approx. | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#95B3D7"|'''Group Companies''' |
|Approx. 180 Subsidiaries | |Approx. 180 Subsidiaries | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#95B3D7"|'''Chairman & CEO''' |
|Tsuyoshi Kikukawa | |Tsuyoshi Kikukawa | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#95B3D7"|'''President & COO''' |
|Michael C. Woodford | |Michael C. Woodford | ||
|- | |- | ||
| − | |bgcolor = "# | + | |bgcolor = "#95B3D7"|'''Consolidated Net Sales (2011)''' |
|847.1 Billion YEN | |847.1 Billion YEN | ||
|} | |} | ||
| Line 59: | Line 59: | ||
|- | |- | ||
|align = "center" rowspan = "2"|'''Revenue''' | |align = "center" rowspan = "2"|'''Revenue''' | ||
| − | |2011: | + | |2011: ¥ 847.10 B / $ 10.58 B |
|- | |- | ||
| − | |2010: ¥ 883. | + | |2010: ¥ 883.08 B / $ 11.03 B |
|- | |- | ||
|align = "center" bgcolor = "#DBE5F1"|Revenue Change | |align = "center" bgcolor = "#DBE5F1"|Revenue Change | ||
| Line 67: | Line 67: | ||
|- | |- | ||
|align = "center" rowspan = "2"|'''Operating Income''' | |align = "center" rowspan = "2"|'''Operating Income''' | ||
| − | |2011: | + | |2011: ¥ 35.4 B / $ 442 M |
|- | |- | ||
| − | |2010: | + | |2010: ¥ 60.1 B / $ 751 M |
|- | |- | ||
|align = "center" bgcolor = "#DBE5F1"|Operating Income Change | |align = "center" bgcolor = "#DBE5F1"|Operating Income Change | ||
| Line 75: | Line 75: | ||
|- | |- | ||
|align = "center" rowspan = "2"|'''Net Profit''' | |align = "center" rowspan = "2"|'''Net Profit''' | ||
| − | |2011: | + | |2011: ¥ 7.38 B / $ 92.25 M |
|- | |- | ||
| − | |2010: | + | |2010: ¥ 47.76 B / $ 597 M |
|- | |- | ||
|align = "center" bgcolor = "#DBE5F1"|Net Profit Change | |align = "center" bgcolor = "#DBE5F1"|Net Profit Change | ||
| Line 83: | Line 83: | ||
|- | |- | ||
|align = "center" rowspan = "2"|'''R&D Expenses''' | |align = "center" rowspan = "2"|'''R&D Expenses''' | ||
| − | |2011: | + | |2011: ¥ 67.28 B / $ 841 M |
|- | |- | ||
| − | |2010: | + | |2010: ¥ 61.85 B / $ 773 M |
|- | |- | ||
|} | |} | ||
| Line 97: | Line 97: | ||
<br> | <br> | ||
Source: SEC fillings | Source: SEC fillings | ||
| + | |||
| + | ===Patents Held by Business group=== | ||
| + | |||
| + | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Business Segment'''</font> | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Medical Systems'''</font> | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Life science & Industrial Systems'''</font> | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Imaging Systems'''</font> | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''R&D/Innovation'''</font> | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Total'''</font> | ||
| + | |- | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Japan patents'''</font> | ||
| + | |align = " center" bgcolor = "#DBE5F1"|2941 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|1067 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|2021 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|1131 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|'''7160''' | ||
| + | |- | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''US patents'''</font> | ||
| + | |align = " center" bgcolor = "#DBE5F1"|1059 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|680 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|1534 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|974 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|'''4247''' | ||
| + | |- | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''China patents'''</font> | ||
| + | |align = " center" bgcolor = "#DBE5F1"|405 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|86 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|444 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|90 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|'''1025''' | ||
| + | |- | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Other patents'''</font> | ||
| + | |align = " center" bgcolor = "#DBE5F1"|948 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|249 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|102 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|165 | ||
| + | |align = " center" bgcolor = "#DBE5F1"|'''1464''' | ||
| + | |- | ||
| + | |bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Total'''</font> | ||
| + | |align = " center" bgcolor = "#DBE5F1"|'''5353''' | ||
| + | |align = " center" bgcolor = "#DBE5F1"|'''2082''' | ||
| + | |align = " center" bgcolor = "#DBE5F1"|'''4101''' | ||
| + | |align = " center" bgcolor = "#DBE5F1"|'''2360''' | ||
| + | |align = " center" bgcolor = "#DBE5F1"|'''13896''' | ||
| + | |- | ||
| + | |} | ||
| + | <br> | ||
| + | |||
| + | In Medical systems business, the company owns 39% of the total number of patents held by the company, with Japanese patents being the highest contributor to that. | ||
==Business Overview== | ==Business Overview== | ||
| Line 106: | Line 156: | ||
| − | + | ||
| − | + | ||
===Organisation Structure=== | ===Organisation Structure=== | ||
| Line 113: | Line 163: | ||
<br> | <br> | ||
| − | In April 2011, the company launched their regional headquarters for Asia and Oceania to coordinate regional strategy and facilitate business infrastructure development. | + | In April 2011, the company launched their regional headquarters for Asia and Oceania to coordinate regional strategy and facilitate business infrastructure development.<br> |
| + | Source: Company website, Linkedin | ||
==Medical Systems Business== | ==Medical Systems Business== | ||
| Line 138: | Line 189: | ||
:(before corporate allocation of expenses) | :(before corporate allocation of expenses) | ||
<br> | <br> | ||
| − | + | {|border="2" cellspacing="0" cellpadding="4" width="81%" align="center" | |
| − | {|border="2" cellspacing="0" cellpadding="4" width=" | + | |
|bgcolor = "#376091"|<font color="#FFFFFF">'''Metrics ( in Billion Yen)'''</font> | |bgcolor = "#376091"|<font color="#FFFFFF">'''Metrics ( in Billion Yen)'''</font> | ||
| + | |bgcolor = "#376091"|<font color="#FFFFFF">''' '''</font> | ||
|align = "right" bgcolor = "#376091"|<font color="#FFFFFF">'''2009'''</font> | |align = "right" bgcolor = "#376091"|<font color="#FFFFFF">'''2009'''</font> | ||
|align = "right" bgcolor = "#376091"|<font color="#FFFFFF">'''2010'''</font> | |align = "right" bgcolor = "#376091"|<font color="#FFFFFF">'''2010'''</font> | ||
|align = "right" bgcolor = "#376091"|<font color="#FFFFFF">'''2011'''</font> | |align = "right" bgcolor = "#376091"|<font color="#FFFFFF">'''2011'''</font> | ||
|- | |- | ||
| − | |bgcolor = "#95B3D7"|'''Medical Systems''' | + | |align = "left" bgcolor = "#95B3D7" rowspan = "3"|'''Medical Systems''' |
| + | |'''Domestic''' | ||
| + | |align = "right"|76.2 | ||
| + | |align = "right"|75.1 | ||
| + | |align = "right"|79.4 | ||
| + | |- | ||
| + | |'''Overseas''' | ||
| + | |align = "right"|307.6 | ||
| + | |align = "right"|275.7 | ||
| + | |align = "right"|275.9 | ||
| + | |- | ||
| + | |bgcolor = "#95B3D7"|'''Total''' | ||
|align = "right" bgcolor = "#95B3D7"|'''383.89''' | |align = "right" bgcolor = "#95B3D7"|'''383.89''' | ||
|align = "right" bgcolor = "#95B3D7"|'''350.75''' | |align = "right" bgcolor = "#95B3D7"|'''350.75''' | ||
|align = "right" bgcolor = "#95B3D7"|'''355.46''' | |align = "right" bgcolor = "#95B3D7"|'''355.46''' | ||
|- | |- | ||
| − | |align = "center" bgcolor = "#DBE5F1"|Endoscopes | + | |align = "center" bgcolor = "#DBE5F1" rowspan = "3"|Endoscopes |
| − | |align = " | + | |align = "center"|Domestic |
| − | |align = "right | + | |align = "right"|44.6 |
| − | |align = "right | + | |align = "right"|40.9 |
| + | |align = "right"|43.8 | ||
|- | |- | ||
| − | |align = "center | + | |align = "center"|Overseas |
| − | |align = "right | + | |align = "right"|168.3 |
| − | |align = "right | + | |align = "right"|151 |
| − | |align = "right | + | |align = "right"|151.6 |
|- | |- | ||
| − | | | + | |align = "center" bgcolor = "#DBE5F1"|'''Total''' |
| − | + | |align = "right" bgcolor = "#DBE5F1"|'''212.9''' | |
| − | + | |align = "right" bgcolor = "#DBE5F1"|'''191.9''' | |
| − | + | |align = "right" bgcolor = "#DBE5F1"|'''195.5''' | |
| − | + | |- | |
| − | | | + | |align = "center" bgcolor = "#DBE5F1" rowspan = "3"|MIP |
| + | |align = "center"|Domestic | ||
| + | |align = "right"|31.6 | ||
| + | |align = "right"|34.2 | ||
| + | |align = "right"|35.6 | ||
| + | |- | ||
| + | |align = "center"|Overseas | ||
| + | |align = "right"|139.3 | ||
| + | |align = "right"|124.6 | ||
| + | |align = "right"|124.3 | ||
| + | |- | ||
| + | |align = "center" bgcolor = "#DBE5F1"|'''Total''' | ||
| + | |align = "right" bgcolor = "#DBE5F1"|'''170.9''' | ||
| + | |align = "right" bgcolor = "#DBE5F1"|'''158.8''' | ||
| + | |align = "right" bgcolor = "#DBE5F1"|'''159.9''' | ||
|- | |- | ||
|} | |} | ||
<br> | <br> | ||
| − | |||
| − | |||
| − | |||
===Projections for FY 2012=== | ===Projections for FY 2012=== | ||
| Line 223: | Line 297: | ||
===Product Portfolio=== | ===Product Portfolio=== | ||
| − | + | The company offers endoscopes in various specialties. The following is a comprehensive list of endoscopes ,according to specialty, that the company offers:<br> | |
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_ebus_bronchoscopes.asp EBUS Bronchoscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_fiber_bronchoscopes.asp Fiber Optic Bronchoscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_video_bronchoscopes.asp Video Bronchoscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_capsulescopes.asp Capsule Endoscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_choledochoscopes.asp Choledochoscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_colonoscopes.asp Colonoscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_duodenoscopes.asp Duodenoscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_echoendoscopes.asp Echoendoscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_enteroscopes.asp Enteroscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_esophagoscopes.asp Esophagoscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_gastroscopes.asp Gastroscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_laryngoscopes.asp Laryngoscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_maf.asp Intubation Scopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_rhinolaryngoscopes.asp Rhinolaryngoscopes] | ||
| + | * [http://www.olympusamerica.com/msg_section/msg_endoscopy_sigmoidoscopes.asp Sigmoidoscopes] | ||
[[Image:prodcuts_olympus.PNG|center|frame| [http://www.olympus-global.com/en/ Olympus]]] | [[Image:prodcuts_olympus.PNG|center|frame| [http://www.olympus-global.com/en/ Olympus]]] | ||
<br> | <br> | ||
| − | |||
| − | |||
| − | |||
| − | |||
===Recent Developments=== | ===Recent Developments=== | ||
<br> | <br> | ||
{|border="2" cellspacing="0" cellpadding="4" width="100%" | {|border="2" cellspacing="0" cellpadding="4" width="100%" | ||
| − | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''S.No | + | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''S.No'''</font> |
| − | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''Date'''</font> | + | |align = "center" bgcolor = "#4F81BD" width="10%"|<font color="#FFFFFF">'''Date'''</font> |
|align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''News/Development'''</font> | |align = "center" bgcolor = "#4F81BD"|<font color="#FFFFFF">'''News/Development'''</font> | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|1 |
| − | |July 2011 | + | |align = "center"|July 2011 |
|[http://www.olympus-global.com/en/news/2011b/nr110704spiruse.html Olympus Corporation to Acquire Spirus Medical, Inc., an endoscope insertion device manufacturer] | |[http://www.olympus-global.com/en/news/2011b/nr110704spiruse.html Olympus Corporation to Acquire Spirus Medical, Inc., an endoscope insertion device manufacturer] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|2 |
| − | |May 2011 | + | |align = "center"|May 2011 |
|[http://www.olympus-global.com/en/news/2011a/nr110526awarde.html Narrow Band Imaging (NBI) endoscopic technology, useful in early cancer detection, selected for the Prime Minister Prize at the 2011 National Commendation for Invention] | |[http://www.olympus-global.com/en/news/2011a/nr110526awarde.html Narrow Band Imaging (NBI) endoscopic technology, useful in early cancer detection, selected for the Prime Minister Prize at the 2011 National Commendation for Invention] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|3 |
| − | |May 2011 | + | |align = "center"|May 2011 |
|[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=851 Olympus ScopeGuide Receives FDA Clearance] | |[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=851 Olympus ScopeGuide Receives FDA Clearance] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|4 |
| − | |April 2011 | + | |align = "center"|April 2011 |
|[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=850 Olympus Names Luke Calcraft as President of Medical Systems Group in the Americas] | |[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=850 Olympus Names Luke Calcraft as President of Medical Systems Group in the Americas] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|5 |
| − | |February 2011 | + | |align = "center"|February 2011 |
|[http://www.olympus-global.com/en/news/2011a/nr110210corpe.html Olympus Names Michael C. Woodford to Serve as President] | |[http://www.olympus-global.com/en/news/2011a/nr110210corpe.html Olympus Names Michael C. Woodford to Serve as President] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|6 |
| − | |October 2010 | + | |align = "center"|October 2010 |
|[http://www.olympus-europa.com/corporate/1696_4717.htm Magnetically guided capsule endoscope system presented at UEGW in Barcelona] | |[http://www.olympus-europa.com/corporate/1696_4717.htm Magnetically guided capsule endoscope system presented at UEGW in Barcelona] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|7 |
| − | |May 2010 | + | |align = "center"|May 2010 |
|[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=762 Olympus Develops World’s Fastest, Most Compatible Endoscope Reprocessor] | |[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=762 Olympus Develops World’s Fastest, Most Compatible Endoscope Reprocessor] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|8 |
| − | |April 2010 | + | |align = "center"|April 2010 |
|[http://www.olympus-europa.com/corporate/1696_4246.htm Olympus Medical Systems Corporation and Siemens Healthcare announce collaborative development of advanced magnetically guided capsule endoscope system for intragastric observation] | |[http://www.olympus-europa.com/corporate/1696_4246.htm Olympus Medical Systems Corporation and Siemens Healthcare announce collaborative development of advanced magnetically guided capsule endoscope system for intragastric observation] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|9 |
| − | |April 2010 | + | |align = "center"|April 2010 |
|[http://www.olympus-global.com/en/news/2010a/nr100412omsiple.html Olympus Medical Systems India Private Limited established, would take care of Sales, marketing and service for medical equipment (endoscopes and related products, and surgical products, etc.)] | |[http://www.olympus-global.com/en/news/2010a/nr100412omsiple.html Olympus Medical Systems India Private Limited established, would take care of Sales, marketing and service for medical equipment (endoscopes and related products, and surgical products, etc.)] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|10 |
| − | |February 2010 | + | |align = "center"|February 2010 |
|[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=745 Olympus Introduces HDTV Gastroscope with Auxiliary Water Channel] | |[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=745 Olympus Introduces HDTV Gastroscope with Auxiliary Water Channel] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|11 |
| − | |November 2009 | + | |align = "center"|November 2009 |
|[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=719 Olympus Introduces World's Smallest GI Scope to Offer 4-Way Angulation and Narrow Band Imaging] | |[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=719 Olympus Introduces World's Smallest GI Scope to Offer 4-Way Angulation and Narrow Band Imaging] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|12 |
| − | |October 2009 | + | |align = "center"|October 2009 |
|[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=713 Improved Endo Capsule Software Enhances Diagnostic Experience] | |[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=713 Improved Endo Capsule Software Enhances Diagnostic Experience] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|13 |
| − | |February 2009 | + | |align = "center"|February 2009 |
|[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=662 Olympus Introduces World’s First Combined Endoscopic Ultrasound Processor for Imaging and Treatment of Digestive and Pulmonary Diseases] | |[http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=662 Olympus Introduces World’s First Combined Endoscopic Ultrasound Processor for Imaging and Treatment of Digestive and Pulmonary Diseases] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|14 |
| − | |December 2008 | + | |align = "center"|December 2008 |
|[http://www.olympus-europa.com/corporate/1696_3032.htm Olympus further enhances credentials by entering into exclusive sales and distribution partnership with market and technology leader Advanced Surgical Concepts] | |[http://www.olympus-europa.com/corporate/1696_3032.htm Olympus further enhances credentials by entering into exclusive sales and distribution partnership with market and technology leader Advanced Surgical Concepts] | ||
|- | |- | ||
| − | |align = " | + | |align = "center"|15 |
| − | |November 2008 | + | |align = "center"|November 2008 |
|[http://www.olympus-europa.com/corporate/1696_2774.htm Olympus Medical Systems Europa and BrainLAB Sign Sales and Marketing Cooperation Agreement] | |[http://www.olympus-europa.com/corporate/1696_2774.htm Olympus Medical Systems Europa and BrainLAB Sign Sales and Marketing Cooperation Agreement] | ||
|- | |- | ||
| Line 322: | Line 407: | ||
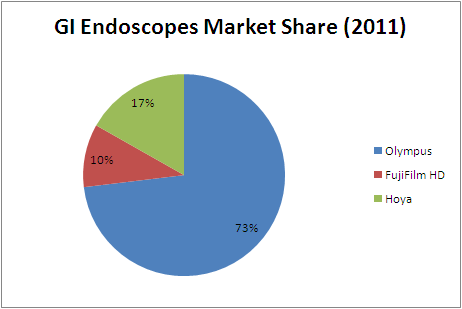
===Market Share=== | ===Market Share=== | ||
| − | [[Image: | + | [[Image:GIshare.PNG|center|frame|Source: Espicom]] |
<br> | <br> | ||
{|border="2" cellspacing="0" cellpadding="4" width="52%" align="center" | {|border="2" cellspacing="0" cellpadding="4" width="52%" align="center" | ||
| Line 408: | Line 493: | ||
* PolyLoop Ligating Loop: Proprietary ligating loop for prophylactic hemostasis | * PolyLoop Ligating Loop: Proprietary ligating loop for prophylactic hemostasis | ||
* Biopsy Forceps: Specially designed to minimize damage to the enteroscope with it's stainless steel construction. | * Biopsy Forceps: Specially designed to minimize damage to the enteroscope with it's stainless steel construction. | ||
| + | |- | ||
| + | |align = "center" rowspan = "1" bgcolor = "#4F81BD"|'''Sigmoidoscopy''' | ||
| + | |bgcolor = "#DBE5F1"|Endoscopic examination, therapy or surgery of the sigmoid flexure | ||
| + | |[[Image:Sig.PNG|100px]] | ||
| + | | | ||
| + | * PCF-S: Easy-reach L/R adapter (MAJ-1072) improves control and comfort for smaller hands and has a soft insertion tube for easier insertion and improved patient comfort. It is compatible with CV-180, CV-160 and CV-140 video processors. | ||
| + | * OSF-V60: 730mm working length gives access to the entire sigmoid colon. It has a Slim, 11.3mm insertion tube provides excellent handling while reducing patient discomfort. | ||
| + | * CF-Q160S: Superior image quality with large display size provides enhanced details throughout the image plane. When used in combination with the company's dedicated flushing pump and CV-160 video system center, a continuously clear view is ensured by a forward water jet that removes obstacles inside the colon at the touch of a switch on the scope. | ||
| + | * OSF-4: 700mm working length facilitates complete insertion. Generous 3.2mm instrument channel accommodates a wide variety of accessories. | ||
|- | |- | ||
|} | |} | ||
| Line 414: | Line 508: | ||
===New Products=== | ===New Products=== | ||
| + | *'''Scope Guide:'''<br>[[Image:Scope.PNG|right|140px]] is an exclusive Olympus technology designed to provide a real-time 3D image of the shape and configuration of the colonoscope inside the body. ScopeGuide assists with finding the optimal location to apply abdominal pressure, easier, more confident scope insertion, and early loop identification. The use of ScopeGuide during colonoscopies may lead to less patient discomfort.<br> By seeing the shape of the entire scope as it moves through the body, ScopeGuide provides additional visual information that is particularly helpful during difficult colonoscopies. The endoscopist can evaluate the extent of looping and get a better sense of which rotational maneuvers will be required to straighten out various loop formations that can occur during colonoscopy. | ||
* '''Evis Lucera Colono-Videoscope:''' It helps in reducing the burden on patients and improving performance for both the inspection and treatment of colorectal cancer through the incorporation of two new functions, namely, high flexibility and highly responsive control at the insertion site, while adopting a thinner scope. | * '''Evis Lucera Colono-Videoscope:''' It helps in reducing the burden on patients and improving performance for both the inspection and treatment of colorectal cancer through the incorporation of two new functions, namely, high flexibility and highly responsive control at the insertion site, while adopting a thinner scope. | ||
* '''Evis Lucera Gastrointestinal Videoscope:''' This slim gastrointestinal videoscope can be inserted through the nasal tract or the mouth to realise high quality images and is suited for the examination of lesions in the upper digestive tract, for example, in the stomach. | * '''Evis Lucera Gastrointestinal Videoscope:''' This slim gastrointestinal videoscope can be inserted through the nasal tract or the mouth to realise high quality images and is suited for the examination of lesions in the upper digestive tract, for example, in the stomach. | ||
| Line 423: | Line 518: | ||
** 6 white LED lights always ensure a clear field of view | ** 6 white LED lights always ensure a clear field of view | ||
** Compact battery with energy efficient technology | ** Compact battery with energy efficient technology | ||
| − | The company is developing technologies to expand the application of capsule endoscopes to the stomach and large bowel. | + | :The company is developing technologies to expand the application of capsule endoscopes to the stomach and large bowel. |
| + | |||
<br> | <br> | ||
Source: Company website, SEC fillings | Source: Company website, SEC fillings | ||
Latest revision as of 03:01, 20 September 2011
Contents
Company overview
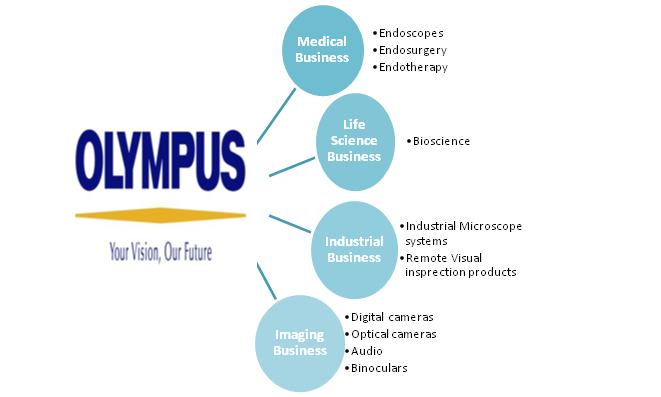
OLYMPUS CORPORATION, a Japan-based manufacturer, mainly manufactures and sells medical products, life and industrial products, imaging products, information communication products and other products. The Company operates in five business segments.
- Medical Systems: Manufacture and sale of medical endoscopes, surgical endoscopes, endoscope disposal equipment and ultrasonic endoscopes
- Life and Industrial: Provides biological microscopes, industrial microscopes, endoscopes for industrial use and non-destructive testing equipment
- Imaging: Manufacture and sale of digital cameras and voice recorders
- Information Communication: Sale of mobile terminals, such as cellular phones
- Others: Development of biomaterials and systems, industrial endoscopes, nondestructive inspection equipment, printers, mobile solutions and mobile contents services, the development and sale of package software, the sale of semiconductor-related equipment and electronic machines, as well as the development of systems
In August 2009, the Company disposed its analytical equipment business to its wholly owned subsidiary, which has been engaged in the manufacturing and sale of clinical laboratory examination equipment. In addition, the Company has sold the subsidiary to Beckman Coulter, Inc. on August 1, 2009. As a result, the Company holds no stake in the subsidiary.
As of March 31, 2011, the Company has 188 subsidiaries and 11 associated companies.
Key Facts
| Established | 1919 |
| Headquarters | Tokyo, Japan |
| Type | Public Company |
| Website | www.olympus-global.com |
| Regional involvement | Worldwide |
| Business lines | Manufacture and Sales of precision machinery and instruments |
| Employees | 34,391 Medical System Business: 15,000 Approx. |
| Group Companies | Approx. 180 Subsidiaries |
| Chairman & CEO | Tsuyoshi Kikukawa |
| President & COO | Michael C. Woodford |
| Consolidated Net Sales (2011) | 847.1 Billion YEN |
Key Financials
| Olympus Corporation | |
| Financial Year End | March, 31st |
| Revenue | 2011: ¥ 847.10 B / $ 10.58 B |
| 2010: ¥ 883.08 B / $ 11.03 B | |
| Revenue Change | -4.07% |
| Operating Income | 2011: ¥ 35.4 B / $ 442 M |
| 2010: ¥ 60.1 B / $ 751 M | |
| Operating Income Change | -41.10% |
| Net Profit | 2011: ¥ 7.38 B / $ 92.25 M |
| 2010: ¥ 47.76 B / $ 597 M | |
| Net Profit Change | -84.45% |
| R&D Expenses | 2011: ¥ 67.28 B / $ 841 M |
| 2010: ¥ 61.85 B / $ 773 M |
Revenue Distribution
Source: SEC fillings
Patents Held by Business group
| Business Segment | Medical Systems | Life science & Industrial Systems | Imaging Systems | R&D/Innovation | Total |
| Japan patents | 2941 | 1067 | 2021 | 1131 | 7160 |
| US patents | 1059 | 680 | 1534 | 974 | 4247 |
| China patents | 405 | 86 | 444 | 90 | 1025 |
| Other patents | 948 | 249 | 102 | 165 | 1464 |
| Total | 5353 | 2082 | 4101 | 2360 | 13896 |
In Medical systems business, the company owns 39% of the total number of patents held by the company, with Japanese patents being the highest contributor to that.
Business Overview
Business lines
The company operates under the following business lines:
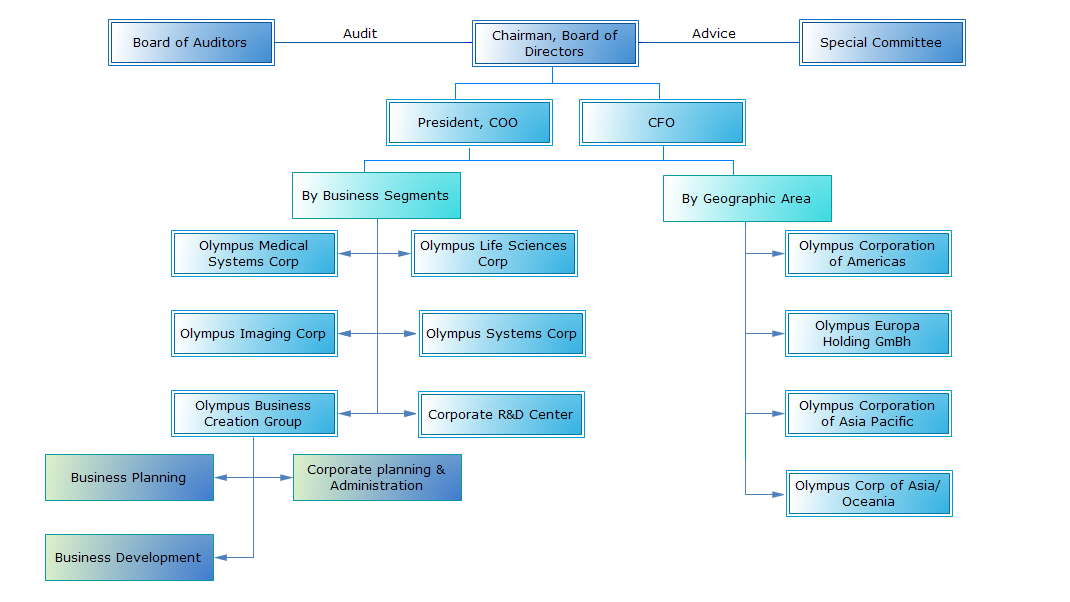
Organisation Structure
In April 2011, the company launched their regional headquarters for Asia and Oceania to coordinate regional strategy and facilitate business infrastructure development.
Source: Company website, Linkedin
Medical Systems Business
The Medical Systems Business covers gastrointestinal endoscopes, surgical endoscopes, endotherapy devices, endoscopic ultrasound systems and medical information systems.
With an approximately 70% share, the Olympus group has secured a leading position in the global Gastrointestinal endoscopy market. Working to expand diagnostic coverage beyond the digestive system, the group is developing new equipment and new techniques ranging from diagnosis through treatment.
It's current research focus is on both minimally invasive treatment technology that reduces the burden on patients and product usability, for example, enhanced performance and functions to benefit doctors and technicians. The group's flagship products are equipped with a high-resolution endoscopic system and Narrow Band Imaging technology so that they can also support cancer diagnosis and treatment.
Medical systems business has the following business lines:
- Endoscopes
Endoscopic video imaging systems, medical information systems, fiberscope systems, broncho endoscope systems, endoscopic ultrasound systems; ultrasound fiberscopes; ultrasound probes; ultrasound centers; ultrasound-guided needle puncture systems; cleaning, disinfecting and sterilization systems; medical treatment peripherals; ancillary products - MIP includes surgical endoscopes and treatment equipments
- Endosurgery
Endoscopy products for gastroenterological surgery, thoracic surgery, urology, gynecology, orthopedic surgery, neurosurgery, cardiovascular surgery, anesthesiology and otolaryngology; treatment and surgical equipment; peripherals - Endotherapy
Endotherapy products
- Endosurgery
Revenue
Medical systems business is the biggest business segment of Olympus corporation:
- Net sales ratio : 40% approx.
- Contribution to total corporate operating income :109% approx.
- (before corporate allocation of expenses)
| Metrics ( in Billion Yen) | 2009 | 2010 | 2011 | |
| Medical Systems | Domestic | 76.2 | 75.1 | 79.4 |
| Overseas | 307.6 | 275.7 | 275.9 | |
| Total | 383.89 | 350.75 | 355.46 | |
| Endoscopes | Domestic | 44.6 | 40.9 | 43.8 |
| Overseas | 168.3 | 151 | 151.6 | |
| Total | 212.9 | 191.9 | 195.5 | |
| MIP | Domestic | 31.6 | 34.2 | 35.6 |
| Overseas | 139.3 | 124.6 | 124.3 | |
| Total | 170.9 | 158.8 | 159.9 |
Projections for FY 2012
Quarter 3
- In endoscopes, new shipments of some products (Lucera) have ground to a halt, but the company expects to see a rebound in 2nd Half. For the year as a whole it projects endoscope sales will be down 5% YoY.
- The company estimates 1st Half sales in the medical systems division at JPY165bn (-6% YoY), and OP at JPY27bn; it projects 2nd Half sales at JPY195bn (+8%) and OP at JPY43bn.
- In the medical systems division, new product launches are planned for both surgical endoscopes and energy devices (treatment tools).
- It projects sales of MIPs (surgical endoscopes, treatment equipment) at JPY180.5bn (+10% YoY). Sales in FY3/11 were flat YoY due mainly to the strength of the yen, but positive growth is expected this year.
- The company projects sales of gastrointestinal endoscopes at JPY164bn, -5% YoY, reflecting production setbacks in 1st Half.
- The earthquake’s impact has caused bottlenecks in purchasing of parts and materials for gastrointestinal endoscopes,leading to delays in product launches. In the latest telephone conference, the company offered no new information about timing. We believe there has been no real change from the company’s comments at the results briefing in May,and it remains to be seen whether the company will be able to launch its new products this fiscal year. The company’s FY3/12 earnings forecasts do not reflect any contribution from new products. Sales in the medical systems division got off to a slow start, falling short of projections in April-May.
Geographic Presence
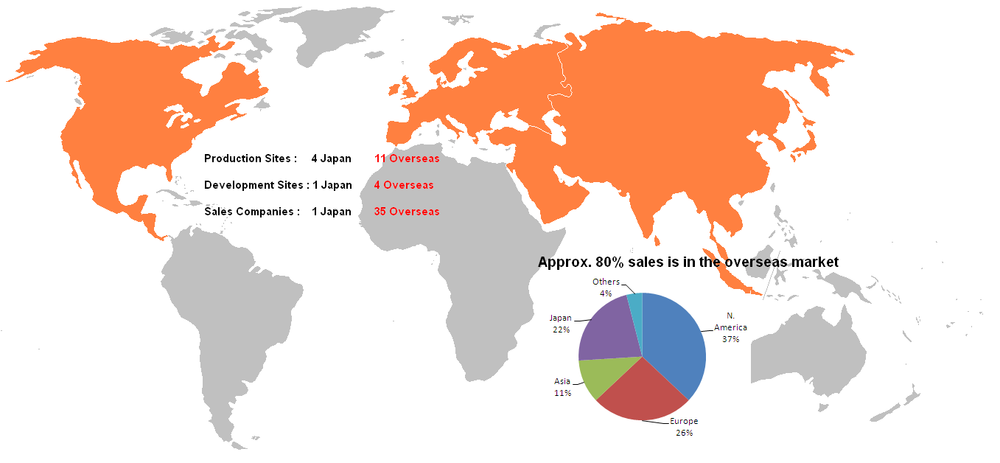
Corporate Strategic Plan
- Expand the endoscopic business base ( Gastrointestinal )
- Gain a dominant market share and ensure profitability in the imaging field
- Early introduction and diffusion of next generation endoscope systems
- Establishment of a significant presence in emerging markets
- Diagnostic technology to promote early detection
- Endoscope technology to achieve definite diagnoses
- Imaging technology for achieving early detection :Narrow Band Imaging (NBI)
- Acquisition of approval of insurance reimbursement for medical effects from early cancer detection using NBI ( in Japan)
- Initiative for standardization of NBI observation as diagnosis
- Gain a dominant market share and ensure profitability in the imaging field
- Growth Drivers
- Expansion of EndoTherapy field
- Introduce Visera Elite, a new integrated endoscopy video system
- Secure a 30% share of the global operating room imaging market in 2015
- Expand the lineup of differentiated products focused on minimally invasive surgical techniques
- Achieve the top share (30%) of the existing global endoscopy market in 2015. Also, secure top share of the Japanese market and further strengthen the sales structure
- Expansion of Chinese and Asian market
- Introduce popularly prices models
- Expand the size of the market and share in such markets. Increase the number of endoscopists and improve their training.
- Expansion of EndoTherapy field
Endoscopes Business
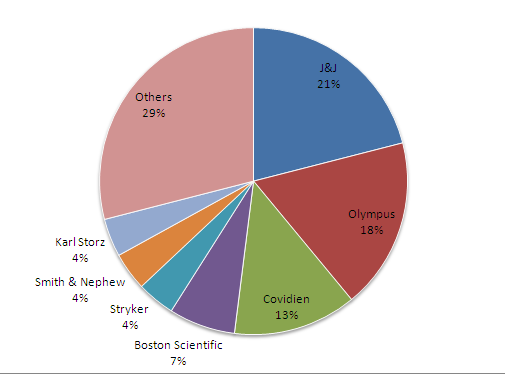
The worldwide endoscopy market (comprising laparoscopy, gastro-intestinal, visualisation, arthroscopy and urology
products, rigid and flexible scopes, implants, surgical and other instruments) was worth approximately US$21.3 billion in 2009. Olympus’ share of this market was estimated to be 18%($3.834B), behind market leader Johnson & Johnson with an
approximate 21% market share. In third place was Covidien (13%), followed by Boston Scientific (7%), Stryker (4%), Smith &
Nephew (4%) and Karl Storz (4%).
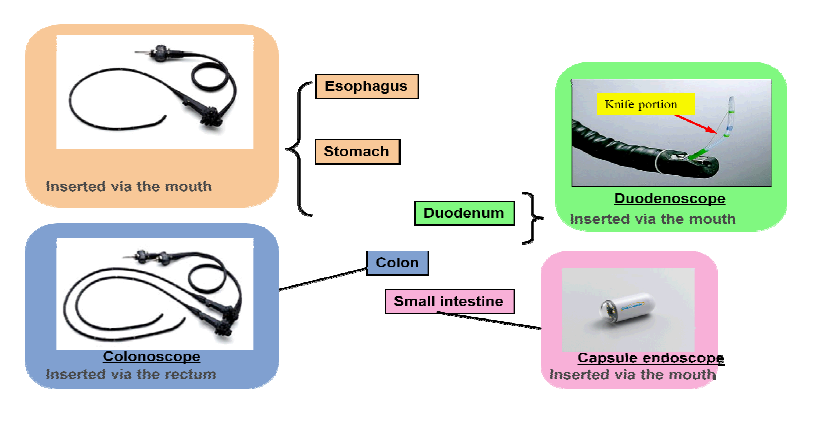
Product Portfolio
The company offers endoscopes in various specialties. The following is a comprehensive list of endoscopes ,according to specialty, that the company offers:
- EBUS Bronchoscopes
- Fiber Optic Bronchoscopes
- Video Bronchoscopes
- Capsule Endoscopes
- Choledochoscopes
- Colonoscopes
- Duodenoscopes
- Echoendoscopes
- Enteroscopes
- Esophagoscopes
- Gastroscopes
- Laryngoscopes
- Intubation Scopes
- Rhinolaryngoscopes
- Sigmoidoscopes
Recent Developments
Gastrointestinal Endoscopes Business
With an approximately 70% global market share for gastrointestinal endoscopes and an established position at the medical vanguard, Olympus maintains its passion for advancing technologies in response to doctors' needs
Diagnosis and Treatment using Gastrointestinal Endoscopy
Diagnosis
- Biopsy
- Cytodiagnosis
- Staining
Treatment
- Cancer (esophageal, stomach, colon, etc.)
- Varcies (esophageal)
- Foreign object retrieval ( esophagus, stomach)
- Hemostasis (stomach)
- Ployps (stomach, colon)
- Gallstones, bile duct stenosis (duodenum)
| Company ( in Billion Yen) | 2009 | 2010 | 2011 |
| Olympus | 212.89 | 191.94 | 195.45 |
| FujiFilm HD | 26.98 | 25.00 | 27.00 |
| Hoya | 48.87 | 47.00 | 45.00 |
Products Offered
Source: Company website
New Products
- Scope Guide:
is an exclusive Olympus technology designed to provide a real-time 3D image of the shape and configuration of the colonoscope inside the body. ScopeGuide assists with finding the optimal location to apply abdominal pressure, easier, more confident scope insertion, and early loop identification. The use of ScopeGuide during colonoscopies may lead to less patient discomfort.
By seeing the shape of the entire scope as it moves through the body, ScopeGuide provides additional visual information that is particularly helpful during difficult colonoscopies. The endoscopist can evaluate the extent of looping and get a better sense of which rotational maneuvers will be required to straighten out various loop formations that can occur during colonoscopy. - Evis Lucera Colono-Videoscope: It helps in reducing the burden on patients and improving performance for both the inspection and treatment of colorectal cancer through the incorporation of two new functions, namely, high flexibility and highly responsive control at the insertion site, while adopting a thinner scope.
- Evis Lucera Gastrointestinal Videoscope: This slim gastrointestinal videoscope can be inserted through the nasal tract or the mouth to realise high quality images and is suited for the examination of lesions in the upper digestive tract, for example, in the stomach.
- Capsule Endoscopes:
They are non-invasive, endoscopic imaging by use of VIDEO CAPSULE ENDOSCOPES to perform examination of the gastrointestinal tract, especially the small bowel. After being introduced in 2007, they have become a revolution. High-resolution imaging, real-time viewing, ease of use and proprietary hard and software make EndoCapsule a comprehensive and powerful system - redefining capsule endoscopy.
One has to simply swallow the Endo capsule, a pill sized (11mm in external diameter and 26mm in length) capsule equipped with a tiny camera and lights, allows pictures to be taken of the entire small bowel. Endo capsule delivers high resolution images equivalent to those provided by endoscopes. EndoCapsule displays a clear and bright field of view for the fine observation of a variety of small bowel abnormalities.
The Real Time Viewer enables the physician to test the proper functionality before the procedure and to confirm the location of the capsule in the GI tract during the process. In addition it is now possible to check whether and when the capsule has left the stomach.
Product Details:- Supersensitive CCD
- Ultra compact lens
- Automatic Brightness Control adjusts illumination to maintain optimal imaging
- A wide depth of field provides superior observation
- 6 white LED lights always ensure a clear field of view
- Compact battery with energy efficient technology
- The company is developing technologies to expand the application of capsule endoscopes to the stomach and large bowel.
Source: Company website, SEC fillings