File:SAR map.gif
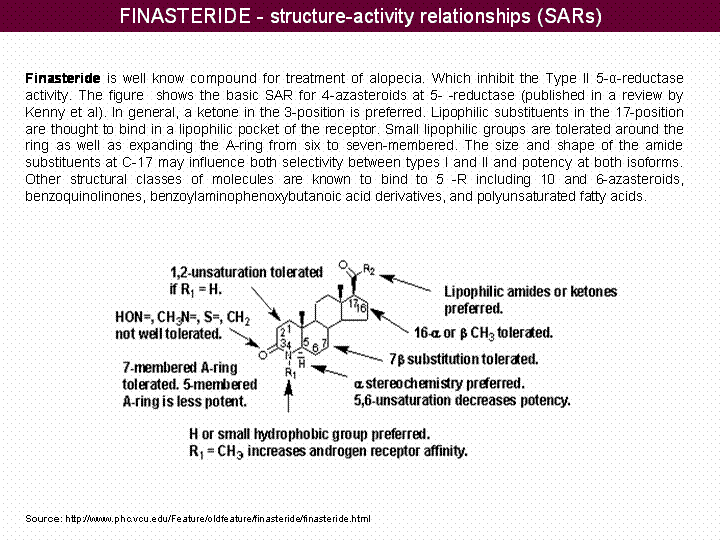
Finasteride is well know compound for treatment of alopecia. Which inhibit the Type II 5-α-reductase activity. The figure shows the basic SAR for 4-azasteroids at 5- -reductase (published in a review by Kenny et al). In general, a ketone in the 3-position is preferred. Lipophilic substituents in the 17-position are thought to bind in a lipophilic pocket of the receptor. Small lipophilic groups are tolerated around the ring as well as expanding the A-ring from six to seven-membered. The size and shape of the amide substituents at C-17 may influence both selectivity between types I and II and potency at both isoforms. Other structural classes of molecules are known to bind to 5 -R including 10 and 6-azasteroids, benzoquinolinones, benzoylaminophenoxybutanoic acid derivatives, and polyunsaturated fatty acids
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 23:08, 9 May 2006 | Error creating thumbnail: /var/www/htdocs/dolcera.com/wiki/includes/limit.sh: line 101: 4093 Aborted /usr/bin/timeout $MW_WALL_CLOCK_LIMIT /bin/bash -c "$1" 3>&- Error code: 134 | 720 × 540 (26 KB) | Vinod.singh@dolcera.com (Talk | contribs) | Finasteride is well know compound for treatment of alopecia. Which inhibit the Type II 5-α-reductase activity. The figure shows the basic SAR for 4-azasteroids at 5- -reductase (published in a review by Kenny et al). In general, a ketone in the 3-positi |
- You cannot overwrite this file.
File usage
The following file is a duplicate of this file (more details):
The following page links to this file: